 You will no doubt be aware of the developments over the Ebola outbreak in Africa. The arrival of a British nurse back in the UK after contracting the virus, in a high-tech plastic tent (LHS), reinforces the fear associated with such diseases. However, it also serves to highlight the ignorance that pervades about infectious diseases: from simple hygiene, through politics and cultural diversity, to our understanding of the molecular basis of viral and bacterial infections. I want to use this Blog however to draw your attention to the lessons we can learn form such devastating diseases and infectious agents.
You will no doubt be aware of the developments over the Ebola outbreak in Africa. The arrival of a British nurse back in the UK after contracting the virus, in a high-tech plastic tent (LHS), reinforces the fear associated with such diseases. However, it also serves to highlight the ignorance that pervades about infectious diseases: from simple hygiene, through politics and cultural diversity, to our understanding of the molecular basis of viral and bacterial infections. I want to use this Blog however to draw your attention to the lessons we can learn form such devastating diseases and infectious agents.  In 1972, Christian B. Anfinsen (RHS) received a share in the Nobel Prize for Chemistry "for his work on ribonuclease, especially concerning the connection between the amino acid sequence and the biologically active conformation". Anfinsen and his colleagues proposed, through a series of elegant experiments, that the information encoded by the primary structure of a polypeptide (i.e. the sequence of amino acids in a protein), determined the three dimensional structure of a given protein. Therefore, if chemical structure determines function, then the amino acid sequence will determine biological function. This is the basis of much of our interpretation of genome data, when we associate the sequence of a gene with its deduced amino acid sequence and then its function. This relationship between primary structure and biological function (or rather conformation) is called the "Thermodynamic hypothesis" or "Anfinsen's dogma" and it has stood the test of time rather well. There are two important caveats: some proteins need a little help along the way to fold into their stable conformation in good time; this is facilitated by a class of proteins called "Chaperones". Secondly, the protein sequence encoded by the gene is not always the same as the sequence of the mature protein, in its functional form. However, I am not going to discuss these two important issues today.
In 1972, Christian B. Anfinsen (RHS) received a share in the Nobel Prize for Chemistry "for his work on ribonuclease, especially concerning the connection between the amino acid sequence and the biologically active conformation". Anfinsen and his colleagues proposed, through a series of elegant experiments, that the information encoded by the primary structure of a polypeptide (i.e. the sequence of amino acids in a protein), determined the three dimensional structure of a given protein. Therefore, if chemical structure determines function, then the amino acid sequence will determine biological function. This is the basis of much of our interpretation of genome data, when we associate the sequence of a gene with its deduced amino acid sequence and then its function. This relationship between primary structure and biological function (or rather conformation) is called the "Thermodynamic hypothesis" or "Anfinsen's dogma" and it has stood the test of time rather well. There are two important caveats: some proteins need a little help along the way to fold into their stable conformation in good time; this is facilitated by a class of proteins called "Chaperones". Secondly, the protein sequence encoded by the gene is not always the same as the sequence of the mature protein, in its functional form. However, I am not going to discuss these two important issues today.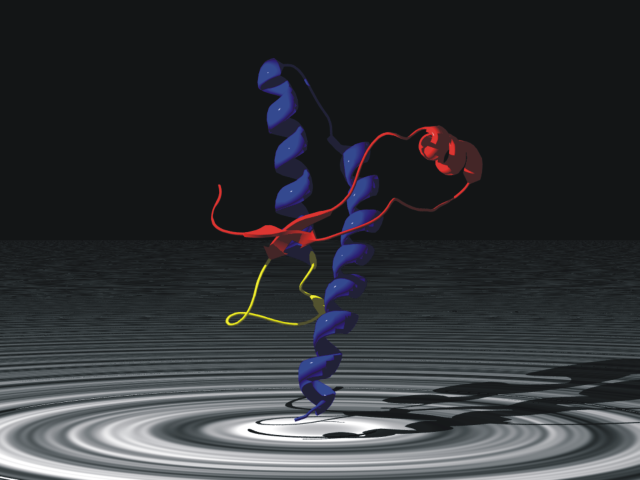 Anfinsen has demonstrated that proteins have a unique conformation that is necessary for function. But what if the protein could adopt two structures with very similar thermodynamic properties? This possibility seems to exist for the prion protein (of mad cow fame). Here, the protein shown right, adopts a stable form which is found in many cells in a normal individual, but which equilibrates to a misfolded (or infectious form) which is also stable. Currently, there is no reason to pin diseases such as scrapie and CJD on anything other than a misfolded protein, which in turn promotes the conversion of the safe prion structure to the infectious form. In short prions defy Anfinsen: there are two acceptable folded states: one is harmless and the other infectious. If we look at the literature in the first half of the 1960s, we also find some landmark work on the ability of proteins to adopt more than one conformation, specifically following the addition of a small molecule. The work of Monod, Wyman and Changeux in France and Koshland Nemethy and Filmer in the US, supported by Max Perutz's elegant structural studies on Haemoglobin at Cambridge, had opened the door for a new way of thinking about proteins and their control (I wont cover post-translational modification here). This work simply shows that a stable conformation can be shifted in the presence of a ligand, but a single (energetically) stable conformation exists in the absence of the ligand (under a give set of conditions). We refer to this as allostery.
Anfinsen has demonstrated that proteins have a unique conformation that is necessary for function. But what if the protein could adopt two structures with very similar thermodynamic properties? This possibility seems to exist for the prion protein (of mad cow fame). Here, the protein shown right, adopts a stable form which is found in many cells in a normal individual, but which equilibrates to a misfolded (or infectious form) which is also stable. Currently, there is no reason to pin diseases such as scrapie and CJD on anything other than a misfolded protein, which in turn promotes the conversion of the safe prion structure to the infectious form. In short prions defy Anfinsen: there are two acceptable folded states: one is harmless and the other infectious. If we look at the literature in the first half of the 1960s, we also find some landmark work on the ability of proteins to adopt more than one conformation, specifically following the addition of a small molecule. The work of Monod, Wyman and Changeux in France and Koshland Nemethy and Filmer in the US, supported by Max Perutz's elegant structural studies on Haemoglobin at Cambridge, had opened the door for a new way of thinking about proteins and their control (I wont cover post-translational modification here). This work simply shows that a stable conformation can be shifted in the presence of a ligand, but a single (energetically) stable conformation exists in the absence of the ligand (under a give set of conditions). We refer to this as allostery. When I heard the news about Ebola, I looked up the work of Erica Ollman Saphire, a name that is both unusual, and memorable! One of my old friends from my early days at Sheffield, now at the Scripps Institute in California had published jointly with Erica. I realised that the protein VP40 (called a viral matrix protein) also defied Anfinsen! Using X-ray crystallography, Saphire's group showed recently in a lovely paper in Cell, that VP40 is capable of forming different stable conformations for the infection and replication stages of its life cycle. Put simply, it is able to economise on function by making use of the same primary structure. Similarities jump out in respect of prion diseases, but also begin to pull the rug away from long in the tooth Biochemists like me! Of course, when you read the work, it becomes clear a priori, that conformational equilibria needn't always be to the left or right, but that the sustainable evolution of protein function will make such phenomena the exception rather than the rule.
When I heard the news about Ebola, I looked up the work of Erica Ollman Saphire, a name that is both unusual, and memorable! One of my old friends from my early days at Sheffield, now at the Scripps Institute in California had published jointly with Erica. I realised that the protein VP40 (called a viral matrix protein) also defied Anfinsen! Using X-ray crystallography, Saphire's group showed recently in a lovely paper in Cell, that VP40 is capable of forming different stable conformations for the infection and replication stages of its life cycle. Put simply, it is able to economise on function by making use of the same primary structure. Similarities jump out in respect of prion diseases, but also begin to pull the rug away from long in the tooth Biochemists like me! Of course, when you read the work, it becomes clear a priori, that conformational equilibria needn't always be to the left or right, but that the sustainable evolution of protein function will make such phenomena the exception rather than the rule.I recall over a year ago Dr Robert Harrison at the Liverpool School of Tropical Medicine telling me how a relatively small number of proteins in snake venom could immobilise an adult in minutes through haematological or neurological mechanisms. I thought this was pretty amazing. But Ebola virus has only 7 proteins with which to disable a human being who will typically express around 20 000 genes in a complex, interrelated and highly regulated manner! By drawing on this knowledge, perhaps we can turn these potential killers into drugs for the elimination of tumours? In the case of ZMapp (shown as a molecular model on the RHS), the antiserum that was supplied in advance of human trials, was produced, somewhat ironically from transgenic tobacco leaves. It is a cocktail of three humanized monoclonal antibodies raised against key viral components. At the moment the details are sensitive, but the fundamental work of Saphire and colleagues elsewhere, as well as the development of plants for the expression of therapeutic antibodies shows how powerful Science can be in facing a health crisis. In my view this is a shining example of how fundamental research on challenging areas of Biology can be justified and must be supported by countries in the developing world. Since we are as a community of Scientists struggling to identify new therapeutic strategies for infectious diseases, work on pathogens should be prioritized, since they have Darwin on their side and can teach us new tricks that could in turn be our salvation!

Thanks for sharing lads. Canadian Animal Lovers
ReplyDelete